
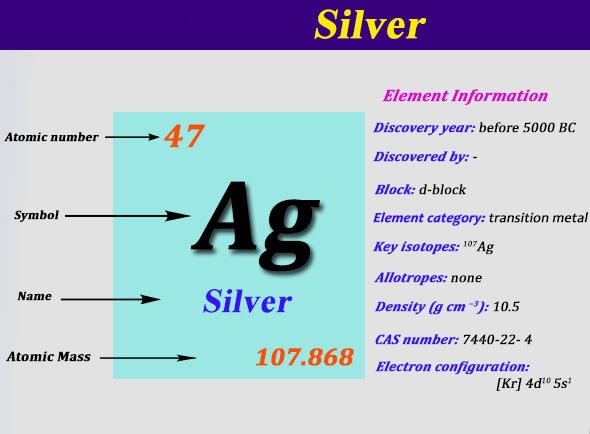
The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10-19 coulombs. Total number of protons in the nucleus is called the atomic number of the atom and is given the symbol Z. A period 5 element is one of the chemical elements in the fifth row (or period) of the periodic table of the chemical elements.The periodic table is laid out in rows to illustrate recurring (periodic) trends in the chemical behaviour of the elements as their atomic number increases: a new row is begun when chemical behaviour begins to repeat, meaning that elements with similar behaviour fall. An ion of platinum has a mass number of 195 and contains 74 electrons. Silver is a chemical element with atomic number 47 which means there are 47 protons in its nucleus. Since the iodine is added as a 1 anion, the number of electrons is 54 53 (1) 54. The number of protons in the nucleus is called the atomic number (Z) and is the property that defines an atom’s elemental identity. Electrons are light particles with a charge of 1 and a mass of 0.00055 amu. You will need to refer to a periodic table for proton values.\)) are useful, because, as you can see, the mass of a proton and the mass of a neutron are almost exactly \(1\) in this unit system. Because the sum of the numbers of protons and neutrons equals the mass number, 127, the number of neutrons is 74 (127 53 74). Neutrons are relatively heavy particles with no charge and a mass of 1.0087 amu. The equations for the half-reactions are then balanced for mass and charge and, if necessary, adjusted so that the number of electrons transferred in each equation is the. For example, iron(II) has a 2+ charge iron(III) a 3+ charge. In this notation, the atomic number is not included. To balance a redox equation using the half-reaction method, the equation is first divided into two half-reactions, one representing oxidation and one representing reduction. Roman numeral notation indicates charge of ion when element commonly forms more than one ion. With a standard atomic weight of circa 1.008, hydrogen is the lightest element on the periodic table. Symbol-mass format for the above atom would be written as Cr-52. Hydrogen is a chemical element with atomic number 1 which means there are 1 protons and 1 electrons in the atomic structure.The chemical symbol for Hydrogen is H. For silver the preeminently important oxidation state in all of its ordinary chemistry is the state +1, although the states +2 and +3 are known.

For an example of this notation, look to the chromium atom shown below:Īnother way to refer to a specific atom is to write the mass number of the atom after the name, separated by a hyphen. The "A" value is written as a superscript while the "Z" value is written as a subscript. The chemical symbol Ag is from the Latin word for silver, argentum (compare Ancient Greek, rgyros), from the Proto-Indo-European root her- (. The usual charge of an element is common to its group. Both the atomic number and mass are written to the left of the chemical symbol. There are four ways to find the charge of an element: Use the periodic table. This is the standard unit for atomic or molecular mass, and 1 amu is thus 1/12 th the mass of a 12 C atom.
The composition of any atom can be illustrated with a shorthand notation called A/Z format. The atomic mass unit (u or amu) is a relative unit based on a carbon-12 atom with six protons and six neutrons, which is assigned an exact value of 12 amus (us).


 0 kommentar(er)
0 kommentar(er)
